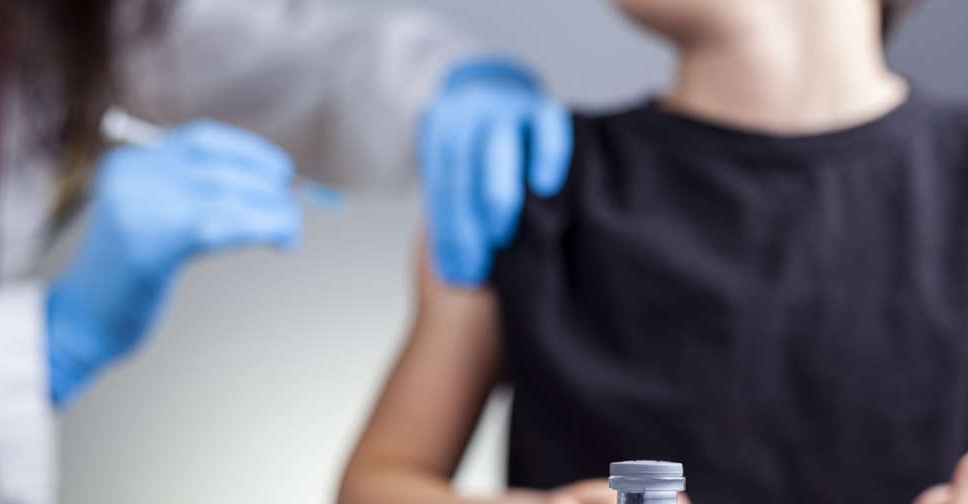
The UAE's Ministry of Health and Prevention has given emergency approval for Sinopharm COVID-19 vaccine for children aged three and above.
In a tweet, the National Emergency Crisis and Disasters Management Authority (NCEMA) said the decision was made after "the recent clinical trials and extensive evaluations, which are in line with the approved regulations".
Ministry of Health approves use of #Sinopharm vaccine for 3-17 age group #WamNews pic.twitter.com/yq8snmccJu
— WAM English (@WAMNEWS_ENG) August 2, 2021
MoHAP announced the provision of Sinopharm vaccine for the age group 3 - 17 The decision comes after clinical trials and extensive evaluations and is based on the emergency use authorization and local evaluations which are in line with the approved regulations.#TogetherWeRecover pic.twitter.com/4Y3VEMMW8V
— NCEMA UAE (@NCEMAUAE) August 2, 2021
It comes after authorities in Abu Dhabi achieved the target of vaccinating 900 children as part of a trial to study the effectiveness of the Sinopharm COVID-19 vaccine in young people.
The Department of Health Abu Dhabi (DoH) said the study examined the vaccine’s effectiveness in reducing the infection rate and severity of symptoms among target groups.
Its preliminary results will be announced at a later stage.




 RTA chalks out route map for Dubai Metro Blue Line
RTA chalks out route map for Dubai Metro Blue Line
 UAE, Cyprus Presidents discuss enhancing strategic partnership
UAE, Cyprus Presidents discuss enhancing strategic partnership
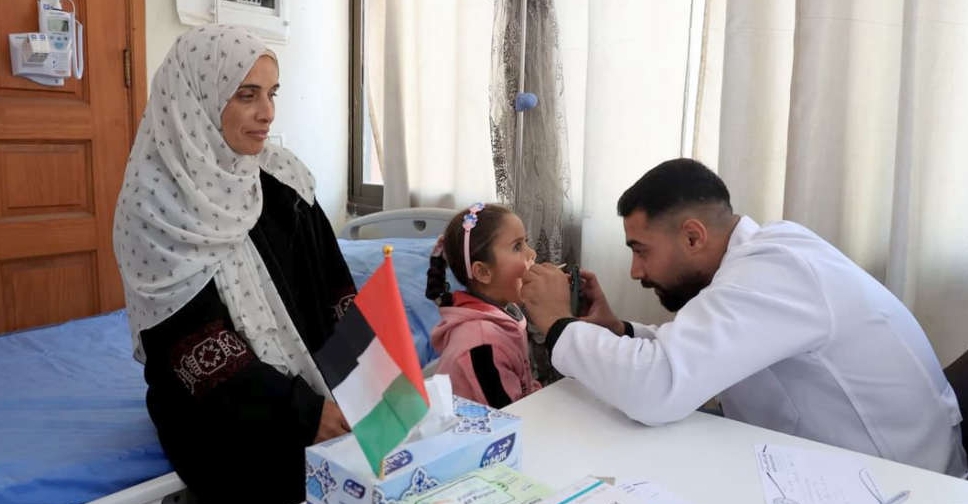 UAE opens Emirates Medical Centre to serve patients in Gaza
UAE opens Emirates Medical Centre to serve patients in Gaza
 UAE President receives official welcome at Presidential Palace in Nicosia
UAE President receives official welcome at Presidential Palace in Nicosia
